Certified primary pH buffers
Read more about our certified pH buffers in the product sheet here.
Buffers certified by the Harned cell method
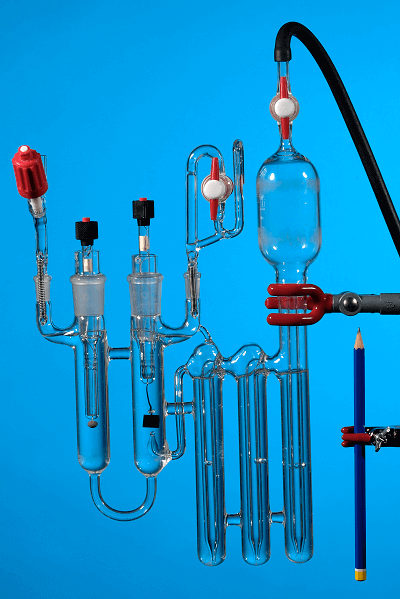
A Harned cell (shown in the picture below) is an electrochemical cell that is used for certification of primary pH buffers. Electrochemical measurements require at least two electrodes. In the Harned cell, the two electrodes are the reversible/standard hydrogen electrode (the rectangular electrode to the right) and a silver/silver chloride electrode.
In the Harned cell the two electrodes are immersed in the same liquid, which is advantageous as there is no junction potential, which would be present in the case of a reduction/oxidation cells connected by a salt bridge, or a liquid junction. There will be a liquid junction potential when there is an interface between two liquids of different composition between the two electrodes. The usual situation for electrochemical measurements is that the reference electrode is in a liquid whose composition deviates from the composition of the liquid in which the other electrode(s) are placed. However, as the two electrodes are in the same liquid the liquid must contain chloride ions in order to utilize with the silver/silver chloride electrode. Thus, chloride must be added to the primary pH buffer for this certification measurement.
The Harned cell method is described in section 4 of R. P. Buck et al., Measurement of pH. Definition, Standards, and Procedures (IUPAC Recommendations 2002), International Union of Pure and Applied Chemistry, Pure and Applied Chemistry, Vol. 74, No. 11, pp. 2169-2200, 2002.
The method requires that measurements are performed in buffer with at least three different concentrations of added chloride, all falling within a specified concentration range. A measurement must also be made in a solution of hydrochloric acid. This measurement can be done independently or simultaneously. DFM do simultaneous measurements in hydrochloric acid and buffer with three different chloride concentrations. The pH of the given batch of buffer is calculated using the measured voltage differences between the two electrodes in each of these four solutions, and using a convention for calculating the activity coefficient of chloride (the effective concentration of chloride ions) the potential difference (pH) of the pH buffer is extracted by extrapolating to zero chloride concentration. The convention used is called the Bates-Guggenheim convention.
The primary buffers fulfill the requirements in section 6.1 of the above-mentioned IUPAC Recommendations (2002). Strict demands to the chemical composition of the buffers are among those requirements. Also, it is specified that two batches of buffer prepared from the same batch of solid salt must have pH values deviating less than 0.003 pH-units from each other.
The pH values stated in the product list below are typical values at 25 °C. The actual pH value of each prepared buffer solution batch is certified, with expanded uncertainties of 0.0016 pH-units between 4 and 9.2, and 0.003 pH-units at 10.0 (carbonate buffer).
The certified pH value of the secondary buffer, shown in the product list below, is determined according to section 8.1 of the above-mentioned IUPAC Recommendations (2002).
The buffers are certified at the temperatures 15 °C, 25 °C, and 37 °C. The certified buffers expire between two and four weeks from the production date as no preservatives or other components are added to prepared batches, and each buffer is only certified once per year.
The certified buffers are used in comparison measurements with secondary or tertiary buffers, respectively, or for calibration of pH electrodes.
The certification is DANAK accredited (accreditation no. 511), according to ISO/IEC 17034:2017.
DFM regularly participates in Key Comparisons through the CCQM, and currently possesses coverage by the CIPM MRA for our two phosphate, phthalate, and carbonate primary pH buffers.
| Product no. | Description |
| R03.101 | Primary pH-buffer ‘Phtalate’ (pH = 4.005) |
| R03.102 | Primary pH buffer ‘Equimolal phosphate (1:1 phosphate)’ (pH=6.865) |
| R03.103 | Primary pH buffer ‘Physiological phosphate (1:3.5 phosphate)’ (pH=7.413) |
| R03.104 | Primary pH-buffer ‘Borate’ (pH=9.180) |
| R03.105 | Primary pH-buffer ‘Carbonate’ (pH=10.012) |
| R03.106 | Secondary pH buffer ‘Sørensen phosphate (1:4 phosphate)’ (pH=7.38) |
You may find further information on prices here.